
STA-Liatest D-Di Plus
VTE is a common condition, affecting about 10 million people worldwide each year, and is a major contributor to global morbidity and mortality. Approximately 25% of deaths globally are related to thrombotic conditions, with about 60% of venous thromboembolism (VTE) cases occurring during or after hospitalization — making hospital-associated thrombosis one of the leading preventable causes of in-hospital deaths.
Early diagnosis and prevention of VTE are critical to reducing the burden of thrombotic diseases for both patients and healthcare systems.
D-Dimer is a fibrin degradation product formed during the process of clot breakdown. The D-Dimer test helps to detect or quantify the level of D-Dimer in the blood.
According to the Vietnam National Heart Association (VNHA, 2022) guidelines on diagnosis, treatment, and prevention of VTE, a high-sensitivity D-Dimer test is recommended to exclude venous thrombosis in patients with low or moderate pretest probability (PTP) without requiring additional imaging tests. (1)
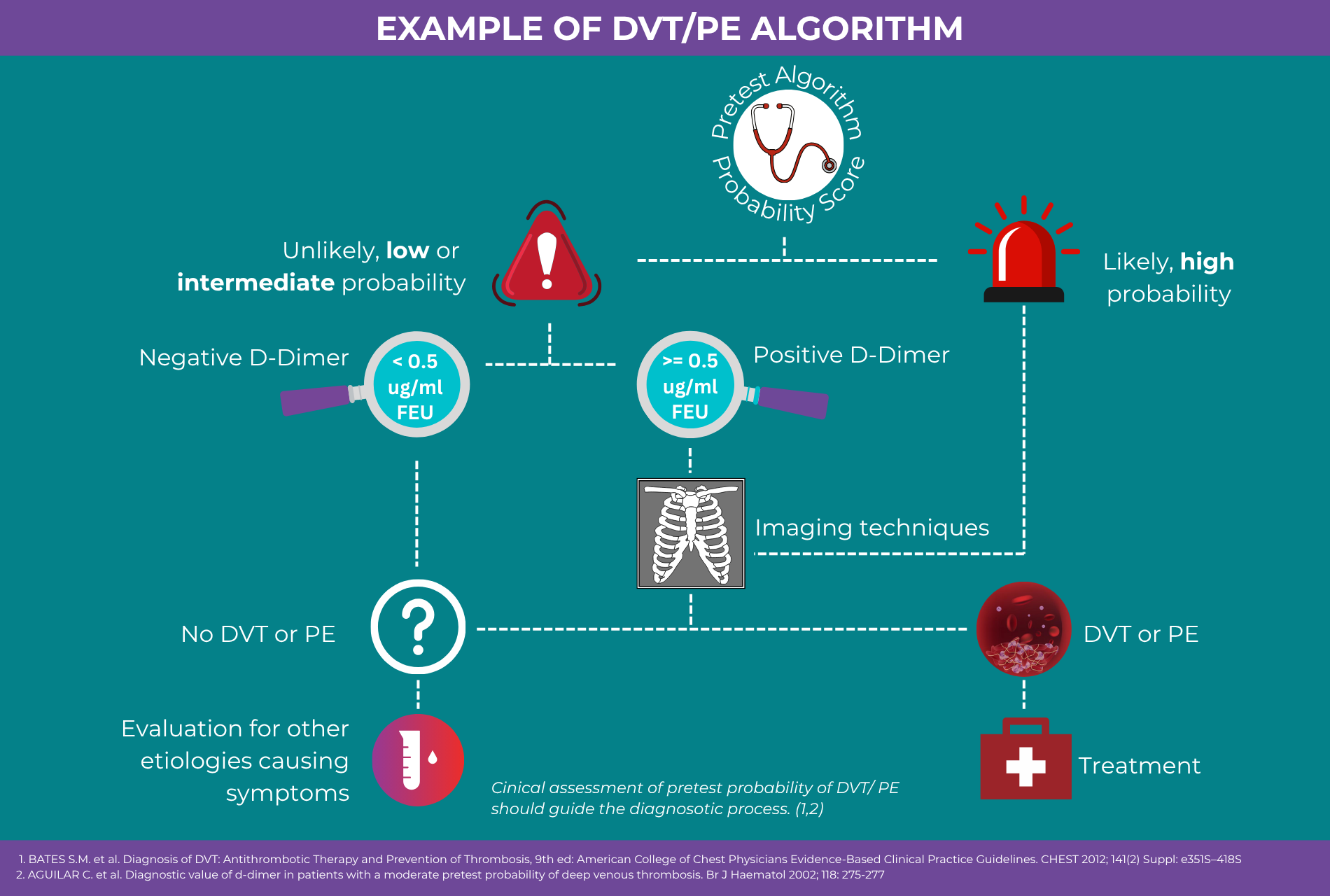
A D-Dimer test used for ruling out VTE should meet the following key criteria:
Sensitivity ≥ 97%
Well-defined and validated cut-off value
High negative predictive value (NPV) ≥ 98%
Rapid turnaround time suitable for emergency settings
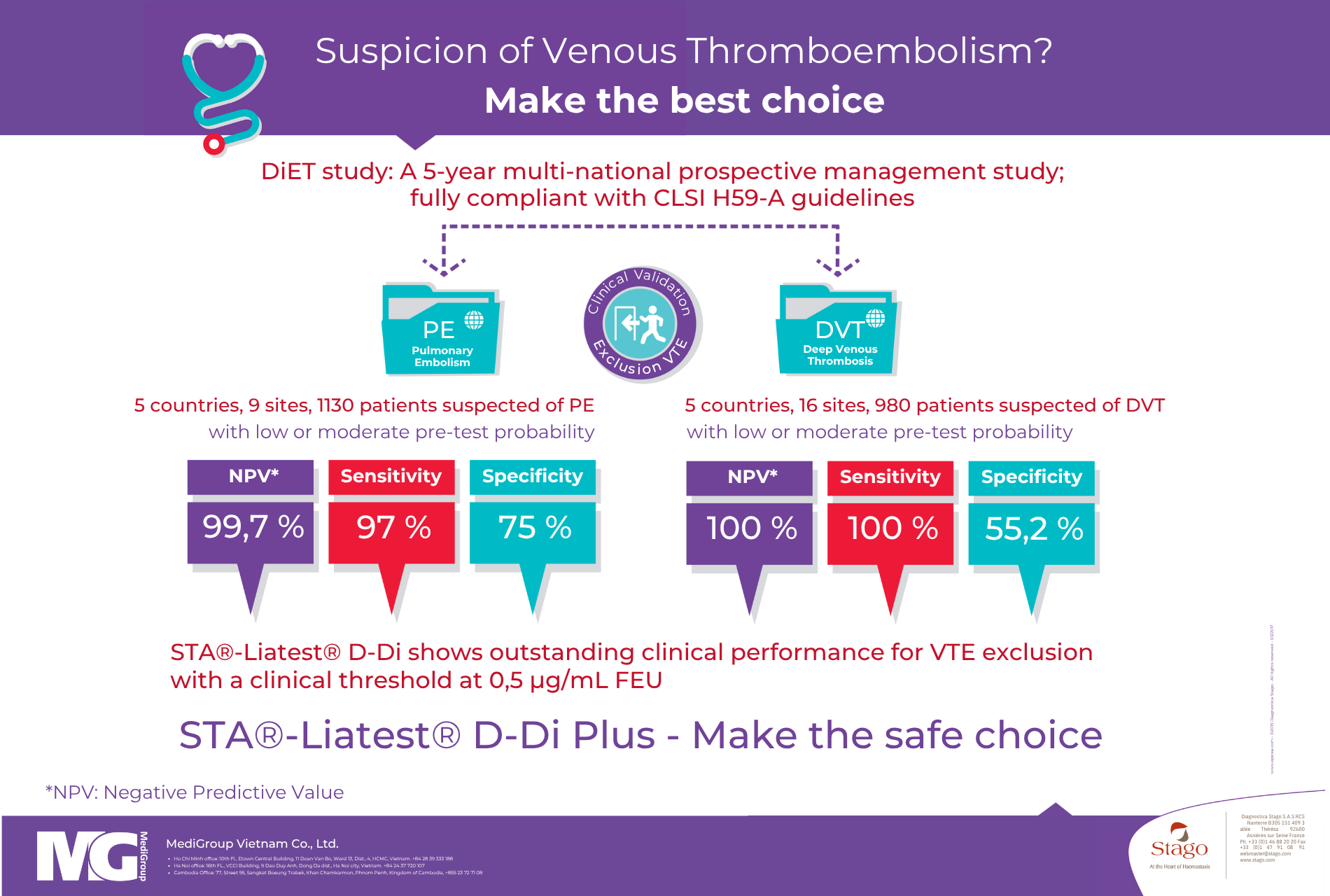
The DiET study confirmed the superior performance of the STA-Liatest D-Di Plus assay in excluding venous thromboembolism (VTE). Conducted across five European centers, the study demonstrated that this test provides (3):
Sensitivity of approximately 97% for deep vein thrombosis (DVT) and pulmonary embolism (PE)
Excellent NPV: 97.6% for DVT and 96.6% for PE
A well-established and validated cut-off value of 0.5 µg/mL FEU
In addition, the test is compatible with STA compact and STA-R analyzers, offering reagent stability of up to 15 days onboard and a rapid result turnaround time of just 7 minutes.
In summary, the STA-Liatest D-Di Plus is a highly effective solution for ruling out venous thromboembolism, supporting timely clinical decisions and playing an important role in VTE management.
Learn more: https://medigroupasia.com/document/d-dimer-line/
Vietnam National Heart Association (VNHA, 2022). Guidelines for diagnosis, treatment, and prevention of venous thromboembolism.
Clinical and Laboratory Standards Institute (CLSI, 2011). Quantitative D-dimer for the exclusion of venous thromboembolic disease; Approved guideline (CLSI document H59-A)
Pernod, G., et al. (2017). Validation of STA-Liatest D-Di assay for exclusion of pulmonary embolism according to the latest CLSI and FDA guidelines. Blood Coagulation & Fibrinolysis, 28(3), 254–260. https://doi.org/10.1097/MBC.0000000000000591